
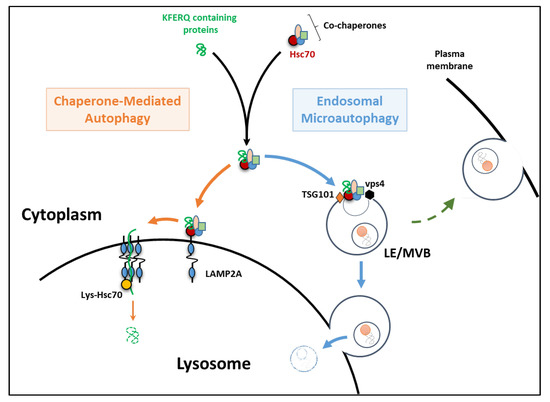
Once generated, stable cell lines are ready to assay however, the process of generating stably transformed cell lines is expensive and time consuming, and assaying different cell types requires the creation of new cell lines. Synthetic fluorescent dyes are popular alternatives to antibodies however, these probes are susceptible to photobleaching and are often found not to be specific for autophagy.Īn alternative to fluorescent dye– and antibody-based monitoring of autophagy is the use of cells transformed with a fluorescent protein chimera (i.e., a biosensor that contains both a fluorescent protein and a functional autophagy marker). The process of chemical fixation itself has been shown to affect cellular structures, leading to potential artifacts and, more importantly, precludes analysis of living cells. All three of these methods have been harnessed to detect autophagy using a variety of fluorescence instrumentation, and, as with all experimental approaches, they are not without caveats.ĭespite the enormous breadth of proteins that can be detected using specific antibodies, immunoassays all require a similar time-consuming protocol that involves fixation, permeabilization, and blocking of the cells, followed by incubation with the primary antibody of choice and potentially a secondary antibody, with wash steps in between. Studying autophagy with fluorescence-based methodsįluorescence-based tools for identifying and monitoring cellular structures or processes include synthetic fluorescent probes, fluorescent protein chimeras ( Figures 1A and 1B), and primary antibodies either labeled with a fluorescent dye or detected with a fluorescent secondary antibody ( Figure 1C). Here we describe current fluorescence-based tools for studying macroautophagy, including the Invitrogen Premo Autophagy Sensors. It is this third process, macroautophagy, that has received the most attention and resulted in the award of the 2016 Nobel Prize in Physiology or Medicine to Yoshinori Ohsumi. Macroautophagy targets a wide range of cargo types (including dysfunctional or unnecessary proteins, organelles, and other cell components) for lysosomal digestion through formation of an autophagosome, an expanding double-membrane organelle that surrounds and engulfs the targeted cellular components and subsequently fuses with the lysosome itself. Chaperone-mediated autophagy utilizes transport mechanisms to transmit cytosolic proteins (containing a specific motif recognized by chaperones) across the lysosomal membrane, where they are degraded by hydrolases in the lumen. Microautophagy requires invagination of the lysosomal membrane to engulf cytoplasmic cargo, which is then delivered into the lysosomal lumen for degradation. The term autophagy covers three cellular processes that all share the lysosome as the ultimate endpoint. Critical to healthy cell functioning, this digestion process serves not only to recycle and repurpose intracellular material but also to dispose of misfolded proteins, damaged organelles, and other superfluous or aberrant entities. (See a list of the products featured in this article.)Īutophagy is a process of cellular self-digestion that involves the segregation and delivery of cytoplasmic cargo for degradation by hydrolytic enzymes through the lysosomal machinery.


 0 kommentar(er)
0 kommentar(er)
